Main menu
You are here
I Didn't Start Believing This Yesterday
I'm finding it hard to believe that it has already been three weeks since I graduated from Queen's. My favourite part of the ceremony by far, was the speech by Emeritus Professor of physics, William McLatchie. This is not just because he mentioned a former student of his, Ted Hsu, the only politician who has ever made me feel thrilled about voting. His speech was unconventional by many standards.
First off, I would expect many graduation speeches to be congratulatory in nature. His was far from it - in fact he said that "mathematics acts as a diode." A much debated claim is that it is easier for the mathematically inclined to follow non-mathematical pursuits than it is for others to do the reverse. But his point was that PhDs who spend their days filling chalk boards with Greek letters - despite their desire to treat non-academics as equals - are regarded by the public as an out-of-touch, nerdy elite. The number of graduands who were on the path to receive a PhD in a quantitative science was rather high, so he felt compelled to tell us what may be a sad truth - that the degrees we would be getting would stigmatize us for the rest of our lives.
Maybe when I have a PhD, no one will want to believe the things I blog about. They might think I'm corrupt enough to put my research before the good of the world. When I try to defend my arguments, they might accuse me of using my academic super-powers to confuse and intimidate. Before that happens, there's something very important that I should mention to you. Even though I am known as quite a stubborn person, I can think of three major topics about which science has convinced me to change my mind:
- I am now suspicious of the merrits of recycling paper.
- I no longer believe that marijuana is a dangerous drug.
- I support nuclear energy.
I want to talk about the last one.
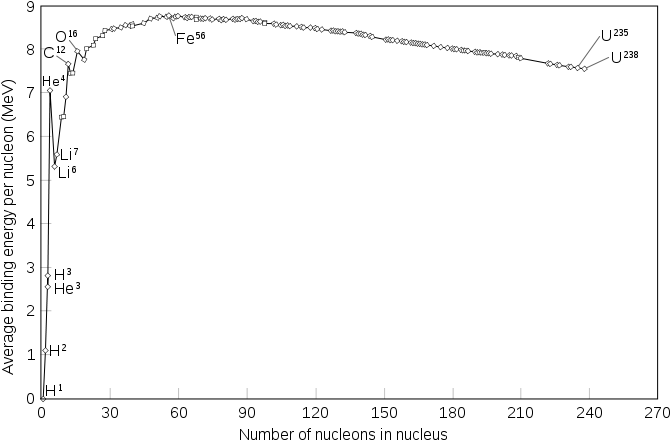
Nuclear power is based upon the desire of matter to occupy a configuration that is more energetically favourable. In some sense, this principle underlies all methods of power generation, but the mechanism for it is unique. Nuclear power plants capture energy that is released not from a chemical reaction (a reaction only involving the electrons) but from a reaction involving the actual nucleus of an atom. The following plot shows how energetic many common nuclei are.
Iron is the most tightly bound nucleus which means it is the most stable. Over time, nuclei on the left side will combine to form more massive nuclei (fusion). The nuclei on the right side decay into daughter nuclei to become more stable (fission). From a nuclear physics standpoint, it looks like we can expect more and more of our universe to be made of iron. Fusion reactors - the types of reactors lampooned in Spider-Man 2 that power stars - hold amazing potential to solve the world's energy crisis but as of yet, no one has come close to building one. All nuclear power plants in the world use a fuel that undergoes fission, usually Uranium-235, and capture the energy that this fuel releases. If someone tells me that he or she is against fusion, I reply with "do your homework" so I'm not even going to touch on why there is nothing wrong with it. When people say they are against nuclear power, they really mean that they are against nuclear fission, so I am going to explain why nuclear fission is a good thing... at least until we develop a working fusion reactor or lower our energy consumption enough to make wind a viable option.
One of the most efficient fossil fuel reactions is the burning of methane (natural gas). The reaction, 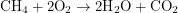 produces about
produces about 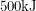 of energy per mole of fuel put in. The main reaction going on inside a nuclear reactor,
of energy per mole of fuel put in. The main reaction going on inside a nuclear reactor, 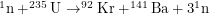 produces
produces 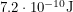 of energy per atom of fuel put in. I calculated this by looking up nuclear masses on a website. To make sure we're comparing apples to apples, it is simple to convert these quantities so that they both give us the amount of energy produced per kilogram of fuel. For methane, it is about
of energy per atom of fuel put in. I calculated this by looking up nuclear masses on a website. To make sure we're comparing apples to apples, it is simple to convert these quantities so that they both give us the amount of energy produced per kilogram of fuel. For methane, it is about 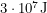 and for uranium, it is about
and for uranium, it is about 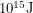 . That's why the amount of carbon dioxide waste required to heat a home for a year is measued in tons whereas the amount of nuclear waste required to do the same thing is measured in grams. But the amount of waste is not the most important factor. What matters is how harmful it is.
. That's why the amount of carbon dioxide waste required to heat a home for a year is measued in tons whereas the amount of nuclear waste required to do the same thing is measured in grams. But the amount of waste is not the most important factor. What matters is how harmful it is.
Most of the uranium in the world is U-238, not U-235 and this is where most of the waste comes from. One way to be more efficient is to use enriched uranium, but this is very expensive. Another is to use a breeder reactor which relies on a completely different set of reactions and is able to use U-238. However, most of the reactors that have received negative media attention use fuel rods that are 99% 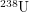 and only 0.7%
and only 0.7% 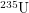 . Spent fuel from these reactors is seen as having unacceptably high levels of Plutonium-239. Notice that the chain reaction
. Spent fuel from these reactors is seen as having unacceptably high levels of Plutonium-239. Notice that the chain reaction 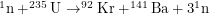 starts with one neutron and produces three neutrons. When one of these neutrons hits a
starts with one neutron and produces three neutrons. When one of these neutrons hits a 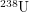 atom, there is a small probability of it becoming an atom of
atom, there is a small probability of it becoming an atom of 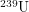 . This unstable atom subsequently beta decays into
. This unstable atom subsequently beta decays into 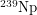 and beta decays again into
and beta decays again into 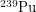 . When one of these nuclear fuel rods is expended, most of what is left is still the original
. When one of these nuclear fuel rods is expended, most of what is left is still the original 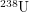 but studies have shown that as much as 0.3% of it can be
but studies have shown that as much as 0.3% of it can be 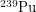 .
.
But how harmful is Pu-239? The first thing we have to keep in mind is conservation of energy. On their journey to become more like iron, nothing we humans can do will change the amount of energy that unstable atoms release into the environment in the form of ionizing radiation. Nuclear reactors just speed up this inevitable process. The relevant question is not how much energy will Pu-239 deposit? but rather how quickly will Pu-239 deposit its energy? This is what really determines how harmful something is. If someone throws a stick at you, every hour on the hour for a year, this will not kill you - it will just be annoying. But if someone were to drop those nearly 10,000 sticks on you at once, this would feel like being crushed by an entire tree. In the same way, nuclear energy trades a nuclide that decays slowly (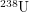 has a half-life of 4 billion years) for one that decays quickly (
has a half-life of 4 billion years) for one that decays quickly (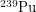 has a half-life of 20,000 years). This is why nuclear waste is more radioactive than the regular rocks in the soil. Just like we can safely say that a falling tree is enough to kill a human, nuclear scientists have figured out an approximate lethal dose of radiation -
has a half-life of 20,000 years). This is why nuclear waste is more radioactive than the regular rocks in the soil. Just like we can safely say that a falling tree is enough to kill a human, nuclear scientists have figured out an approximate lethal dose of radiation - 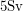 annually, which means 5 Joules per kilogram of body tissue over the course of a year. To see how much energy Pu-239 emits with each decay, we will be good nuclear physicists and look at the energy spectrum.
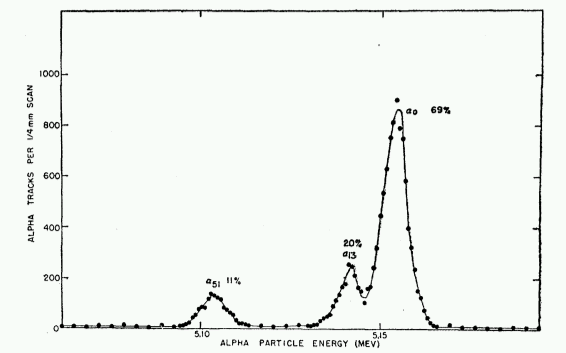
annually, which means 5 Joules per kilogram of body tissue over the course of a year. To see how much energy Pu-239 emits with each decay, we will be good nuclear physicists and look at the energy spectrum.
Like many nice sources, Plutonium-239 has a well defined peak. Most of the ionizing particles (alpha particles) that get emitted will have an energy of 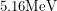 which is
which is 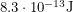 . We can use the half-life and the laws of exponential decay to say that if we have
. We can use the half-life and the laws of exponential decay to say that if we have 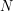 atoms of Pu-239 in a collection of waste,
atoms of Pu-239 in a collection of waste, 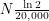 of them will decay each year. This means that we get
of them will decay each year. This means that we get 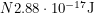 of energy emitted each year. Using the fact that a light human weighs about
of energy emitted each year. Using the fact that a light human weighs about 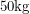 this amounts to
this amounts to 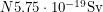 . If you stand 10 metres away from a pile of Pu-239, you are absorbing 0.1% of the alphas coming out of it because the surface area of a human,
. If you stand 10 metres away from a pile of Pu-239, you are absorbing 0.1% of the alphas coming out of it because the surface area of a human, 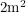 is 0.002 times the surface area of a sphere having that radius and only the "front half" of your surface area is exposed. This means
is 0.002 times the surface area of a sphere having that radius and only the "front half" of your surface area is exposed. This means 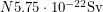 is the effective dose. To reach the lethal dose, the number
is the effective dose. To reach the lethal dose, the number 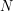 would have to be
would have to be 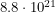 atoms. After substituting the mass of Pu-239 and using the 0.3% rule, this amounts to around
atoms. After substituting the mass of Pu-239 and using the 0.3% rule, this amounts to around 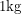 of spent fuel. You can have a salt shaker of spent fuel, fresh from the reactor (enough to have heated your home for a year), in your kitchen and not die.
of spent fuel. You can have a salt shaker of spent fuel, fresh from the reactor (enough to have heated your home for a year), in your kitchen and not die.
In the rough calculation we have also assumed that the alpha particles travel through a vacuum. In reality, they interact with air molecules along the way. The shielding provided by the air is enough that they become essentially harmless after a few centimetres. Contrary to popular belief, nuclear power plants don't release their waste into the environment where people can easily come within 10 metres of it and have to rely on the shielding of the air. A few years ago, I went on a tour of Point LePreau Generating Station in New Brunswick and saw that they had 28,000 tons of spent fuel encased in concrete. An oil company would have produced 80 million tons of carbon dioxide to produce the same amount of electricity and it would be loose in the atmosphere. Instead all of the waste the 25 year old plant has ever produced is isolated from the environment and kept under constant surveillance.
So if this means that nuclear power is safe under normal operations, what about accidents? If you look at the U-235 reaction, one neutron goes in and three neutrons come out. This means that with improper moderating of the neutrons, the reaction can proceed quickly enough to cause a nuclear meltdown. Most people would agree that the most deadly nuclear meltdown that ever occurred, happened at the poorly designed reactor - Chernobyl. While the effects of this disaster were terrible, a disgruntled old man famous environmentalist argues that no more than 75 people died of acute radiation poisoning, in his book, The Revenge of Gaia. Anyone who says that thousands of people died is attributing two decades worth of shortened lifespans to Chernobyl, that are only circumstantially linked to it. The Wikipedia article on Chernobyl is right when it says that "estimates vary enormously." I cannot tell if Lovelock's numbers are accurate, but what I can tell is that nuclear power is still one of the safest sources of energy. A famous study by ExternE found that burning coal causes 4,000 times as many deaths per TWh of energy as the nuclear industry, even when the Chernobyl disaster is taken into account. Of course this study does not account for the recent tragedy at Fukushima I, but even if it did, this disaster would have to be several thousand times worse than Chernobyl to make up the difference. This led me to an interesting article comparing the two.

Nuclear power does not only beat fossil fuels in terms of safety, it also beats them in terms of nuclear waste! The shale surrounding fossil fuel deposits is more radioactive than the average rocks exposed to people every day. Because of the sheer amount of mining that must be done to get useful amounts of energy out of coal it contributes more nuclear waste to the environment than the entire nuclear industry. Knowing that the effects of nuclear energy are much less severe, Lovelock volunteered to have small amounts of nuclear waste dumped on his lawn. He has even tried this himself and observed that plants thrive in areas that have been lightly irradiated. Organizations like Greenpeace campaign against nuclear power claiming that it is unnatural. In fact, natural nuclear reactors using the very same fuel as our own are known to have formed in prehistoric times. There are even some who think that they assisted the evolution of life on Earth. Indeed, co-founder of Greenpeace, Patrick Moore is pro-nuclear. He left the group after feeling that his views had been co-opted by fanatics who are more interested in stopping people from doing science.
The world's energy consumption is only going to increase and we need an energy source that is clean and plentiful without requiring the right kind of weather to work. Even if we don't invest in fusion, breeder reactors or clever methods of reporcessing spent fuel, the amount of uranium in the Earth (and the ocean) is enough to sustain the world's projected energy demand for thousands of years. Whenever a politician says that we should "abandon nuclear in favour of wind and solar," they really mean "attempt to use wind and solar energy to replace nuclear power plants but mostly use oil and gas when the renewables inevitably fall short." This is especially true of Angela Merkel's sudden change of heart to end nuclear power in Germany. Not only has her government started using fossil fuels to make up the demand, they have imported nuclear energy from France. So you can say "not in my backyard" all you want. Without nuclear reactors somewhere in the world, I expect that meeting your energy demand will be as hard as meeting your demand for medical isotopes.
So ends this treatise. Did I used to think nuclear power was evil? Of course! What other reaction is there when someone tells you that a power plant using the same technology as the atomic bomb is dumping toxic waste all over the world? I have since amended my views and unlike Greenpeace, I did that using science. Because after all... it works, bitches.